Background:
Hereditary thrombotic thrombocytopenic purpura (hTTP), or Upshaw-Schulman syndrome, is a rare thrombotic microangiopathy due to a severe congenital deficiency of ADAMTS13 resulting from bi-allelic ADAMTS13 mutations. As in other plasma factor deficiencies, treatment consists of replacement of the missing ADAMTS13, which can currently be attempted with fresh frozen plasma, solvent/detergent plasma or plasma-derived factor VIII/Von Willebrand factor concentrates. Although prophylactic plasma infusions prevent acute episodes in many but not all hTTP patients, current treatment guidelines state conditional recommendations for prophylaxis outside of pregnancy, because evidence supporting the burdensome plasma prophylaxis in hTTP patients is scarce.
Aims:
Prospective evaluation of the incidence of acute episodes occurring in hTTP patients during periods with and without prophylactic plasma treatment.
Methods:
All patients with a confirmed diagnosis of hTTP enrolled in the International hTTP Registry between January 01, 2006 and December 31, 2021 were eligible for study. Diagnosis of hTTP was based on persisting ADAMTS13 activity <10%, bi-allelic ADAMTS13 mutations, and/or an infusion trial with full recovery and a half-life of plasma ADAMTS13 of around 60 hours. We recorded the patients' detailed medical history at enrollment and then collected prospective data on the clinical course, treatment modalities and laboratory values through site-visits and case report forms completed by treating physicians during annual visits or at occurrence of acute TTP episodes. Patients were followed until December 31, 2022 (database lock), death, or loss to follow-up, whatever came first. This post-hoc analysis evaluated acute TTP episodes during follow-up, stratified by periods on prophylaxis and without prophylaxis. Patients could contribute follow-up time to both periods (strata), but never simultaneously and gestation periods were excluded. Minimum criterion for long-term prophylaxis was defined as at least one monthly plasma infusion over a period of ≥ 6 months. Results were reported using descriptive statistics without formal statistical comparison and incident rates of acute TTP events were calculated per 1000 person-years with corresponding 95% confidence intervals (CIs).
Results:
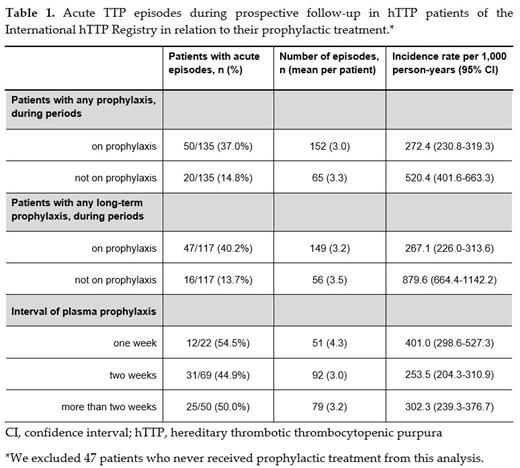
At the end of December 2021, the International hTTP Registry had 182 confirmed hTTP patients eligible for this analysis that were followed for a median period of 3.6 (range, 0-18.5) years. The gender distribution showed more female (n=98, 53.8%) than male (n= 84, 46.2%) patients enrolled, a disparity likely related to a surge of diagnosis in women due to pregnancy complications. During prospective follow-up, 47 (25.8%) hTTP patients never had prophylactic treatment, while 135 (74.2%) received any form of prophylactic treatment, including long-term prophylaxis in 117 (86.7%). Patients who received any prophylactic treatment during follow-up had an incidence rate (95% CI) of acute TTP episodes of 272.4 (230.8-319.3) per 1000 person-years while receiving prophylaxis and of 520.4 (401.6-663.3) per 1000 person-years during periods without prophylaxis (Table 1). In the patients on long-term prophylaxis, the incidence rate of acute TTP episodes was 267.1 (226.0-313.6) per 1000 person-years in periods with prophylaxis, and 879.6 (664.4-1142.2) per 1000 person-years without prophylaxis. Irrespective of treatment interval, about half of patients on prophylaxis had break-through events, with the highest incidence rate observed in the group of patients receiving plasma products weekly, possibly reflecting their more precarious or severe disease course.
Conclusions:
In this large hTTP patient cohort observed for five years on average, we confirm the incidence rate of acute events as reported by Tarasco et al. (Blood 2021;137;3563-75). Here, we demonstrate for the first time that hTTP patients with disease courses requiring prophylaxis benefit from this burdensome treatment, which was most evident in patients on long-term prophylaxis. Current treatment options are however insufficient to suppress residual disease activity leading to break-through events observed in half of the patients on prophylaxis. Novel therapies, such as recombinant ADAMTS13, with the potential of reaching higher plasma levels, will likely overcome limitations of current treatment options.
Disclosures
Schraner:NovoNordisk: Other: Congress Travel Support. Tarasco:SOBI: Other: Congress Travel Support; NovoNordisk: Other: Congress Travel Support. Friedman:Bayer: Consultancy; CSL Behring: Honoraria; NovoNordisk: Consultancy; Gentech: Consultancy; Sanofi: Consultancy; Siemens: Honoraria; Werfen: Consultancy, Honoraria. Hrachovinova:Takeda: Honoraria. Knöbl:NovoNordisc: Honoraria, Research Funding; Ablynx/SOBI: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Technoclone: Honoraria; Alexion: Honoraria; Biotest: Honoraria; CSL Behring: Honoraria. Matsumoto:Chugai Pharmaceutical: Research Funding; Alexion Pharma: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Asahikasei Pharma: Research Funding, Speakers Bureau; Sanofi: Consultancy, Research Funding, Speakers Bureau. Von Krogh:Sanofi Genzyme: Membership on an entity's Board of Directors or advisory committees. Sakai:Takeda: Research Funding. Windyga:AlfaSigma: Honoraria; Alynylam Pharmaceuticals: Honoraria; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL Behring: Honoraria; Bayer AG: Honoraria; NovoNordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Octapharm: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi/Genzyme: Honoraria, Research Funding; Shire/Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sobi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Swixx Biopharma: Honoraria. Lämmle:Baxalta/Takeda: Other: Chairman for data safety monitoring (Baxalta 281102) and Takeda (TAK 755-3002); Sanofi: Other: Chairman for data safety monitoring. Kremer Hovinga:Roche: Other: Lecture Fees; Ablynx/Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SOBI: Other: Congress Travel Support; Baxter/Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal